Hans-Ulrich Demuth
Fraunhofer Institute for Cell Therapy and Immunology, Germany
Title: Targeting Neurotoxic N-Terminal Pyroglutamated Abeta (Pgluaβ) with inhibitors of Glutaminyl Cyclase (Qc) and Pgluaβ-specific antibodies has reached clinical stage
Biography
Biography: Hans-Ulrich Demuth
Abstract
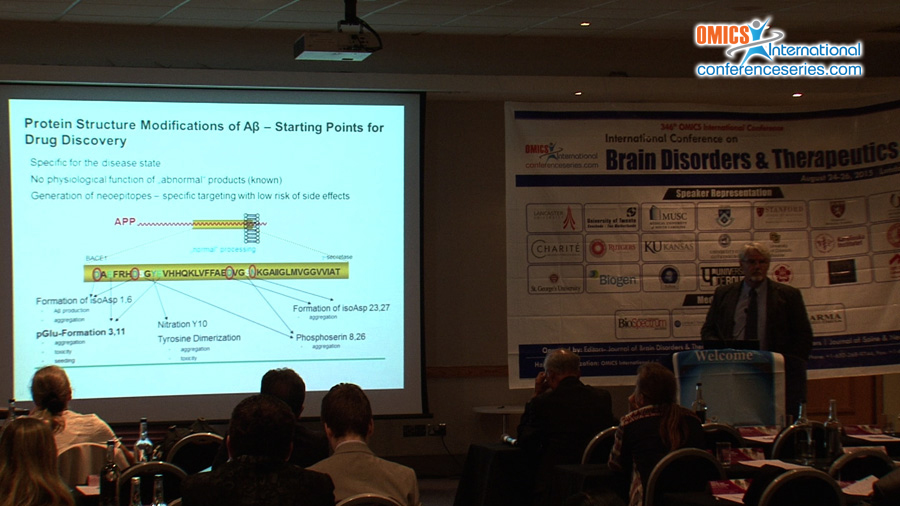
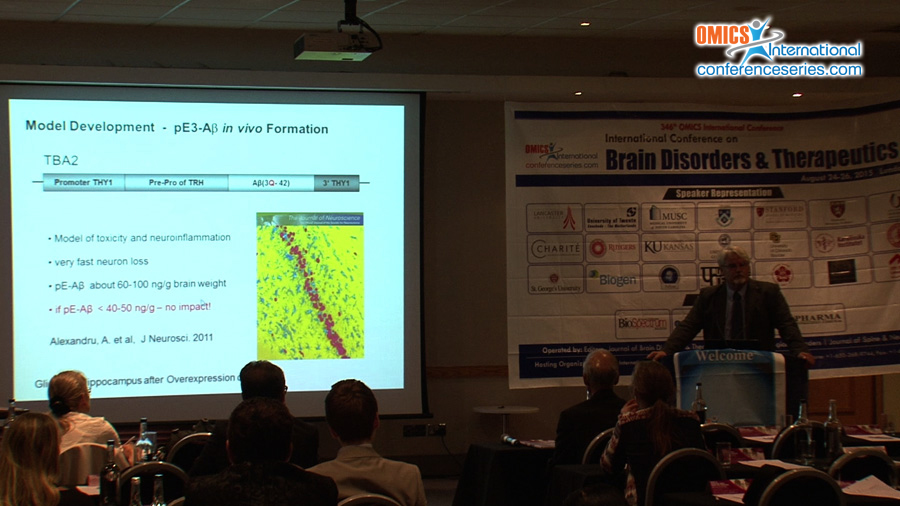
Alzheimer’s disease (AD) is characterized by neuron-loss and neuro-inflammation. Although N-truncated and N-pyroglutamated Aβ-peptides (pGluAβ) are known as prominent constituents of plaques in AD-brain, their importance was overseen and pathways leading to their formation not understood. Because of their abundance, resistance to proteolysis, such N-terminally modified peptides can be important for initiation of pathological cascades leading to AD. Our work uncovers, that N-terminal pGluAβ-formation is catalyzed by QC1. QC-expression is upregulated in the cortex of individuals with AD and correlated with the appearance of pGlu-modified Aβ. Oral application of QC-inhibitors resulted in reduced pGlu3Aβ42 burden, but surprisingly also to the attenuation of the 1.000fold higher amounts of total Aβ in transgenic AD-models1. These observations led to the hypothesis that pGluAβ can seed Aβ-oligomerization by self- and co-aggregation with other monomeric Aβ-species2. Amounts of less than 10nM pGlu3Aβ42 generated cytotoxic oligomers which are over 20 fold more stable than oligomers of “classical†full-length Aβ-peptides. Such mixed pGlu3Aβ42-oligomers propagate their toxic structure in a prion-like manner. Moreover, the neurotoxicity unfolds to be tau-dependent in cell culture as well as in animal models. There, specific neuronal expression of pGluAβ provides in vivo evidence for profound pGluAβ neurotoxicity and gliosis induction2. Hence, a drug development program was entering regulatory testing 2010. Two phase 1: Single (SAD) and multiple ascending dose (MAD) trials of the compound PQ912 in healthy volunteers were conducted. They revealed PQ912 safe and well tolerated. Dose-proportional pharmacokinetics and a strong pharmacodynamic relationship were observed in plasma and CSF justifying studies involving patients. PQ912 is the first QC-inhibitor for treatment of AD since March 2015 in phase 2. Our research concentrates further on posttranslational modifications of Aβ leading to alternative pathways of the turnover of precursor protein APP.