Nan-Shan Chang
National Cheng Kung University,Taiwan
Title: Tumor suppressor WWOX limits neurodegeneration and Zfra mitigates the symptoms of Alzheimer’s disease
Biography
Biography: Nan-Shan Chang
Abstract
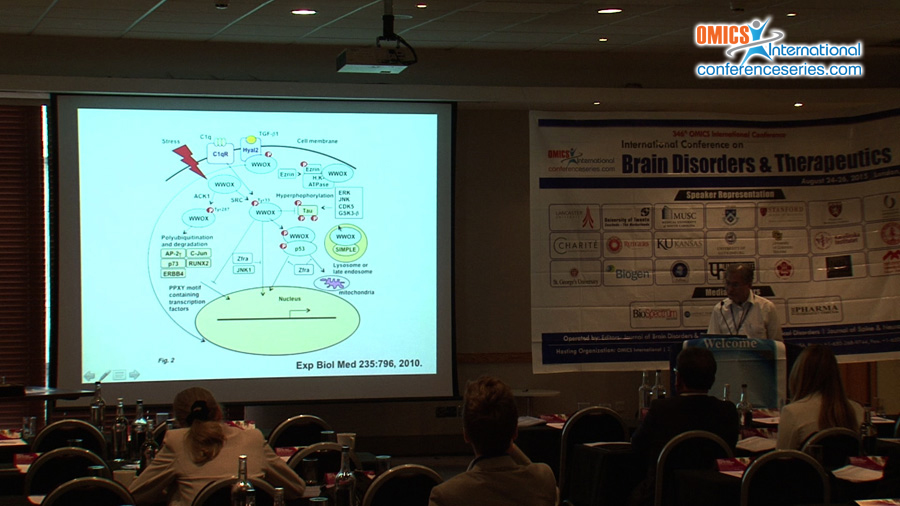
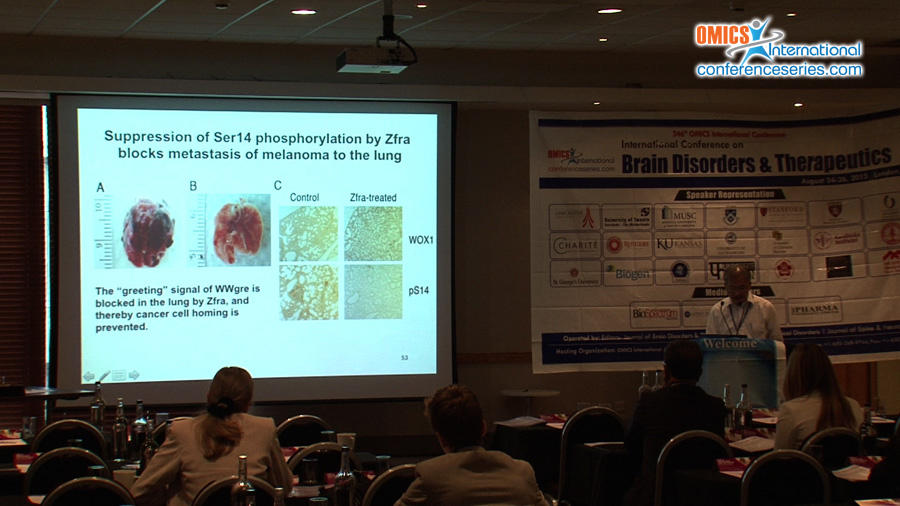
Substantial evidence reveals that tumor suppressor WW domain-containing oxidoreductase (known as WWOX, FOR or WOX1) blocks the progression of Alzheimer’s disease (AD). We have first demonstrated that WWOX binds tau and tau-hyperphosphorylating enzymes GSK3ï¢, ERK and JNK1, and thereby inhibits tau hyperphosphorylation (JBC 279:30498, 2004; Cell Death Differ 19:1049, 2012). In addition, WWOX promotes neuronal differentiation. Unfortunately, WWOX is frequently downregulated in the AD hippocampi (JBC 279:30498, 2004). And, this downregulation results in spontaneous aggregation of TIAF1 and TRAPPC6Aï„, which leads to caspase activation, tau aggregation, APP degradation, formation of amyloid ï¢ and plaques in humans and in Wwox gene knockout mice (Cell Death Dis 1:e110, 2010; Oncotarget 6:3578, 2015). When WWOX protein is lost due to alteration of WWOX/Wwox gene such as missense or nonsense mutation and deletion, this leads to ataxia, epilepsy, dementia, neurodegeneration, diseases associated with HDL lipid metabolism, and early death in humans, mice and rats (Oncotarget 5:11792, 2014; Exp Biol Med 240:400, 2015). Our studies revealed that significant upregulation of proteins, which are involved in neurodegeneration, is found in the cortex and hippocampus of Wwox gene knockout mice. These proteins include GSK3ï„, ERK, pERK, JNK1, pJNK1, TIAF1, pS35-TIAF1, TRAPPC6Aï„, pS37-TRAPPC6Aï„, ï¡-synuclein, C9orf72, TDP-43 and others. Notably, a zinc finger-like peptide Zfra is able to ameliorate the symptoms of AD in the triple transgenic mice, including neuronal death and memory and learning capabilities, suggesting its potential use in therapy for AD. (supported by DoD, USA and MOST and NHRI, Taiwan)